Introduction: For patients (pts) with relapsed or refractory acute myeloid leukemia (r/r AML) allogeneic stem cell transplantation (ASCT) has become an established highly effective and potential curative treatment option. Even for pts with active disease, direct ASCT using sequential conditioning regimens has shown promising outcomes. In this retrospective study, we examined prognostic factors for long-term survival in r/r AML pts with active disease with special focus to the prognostic value of blast clearance during condition therapy.
Patients and Methods: 186 r/r AML pts treated with sequential conditioning regimens at our center between 2014-2023 were included in our analysis. Patients underwent high-dose melphalan (100 - 140 mg/m 2, day -11 prior ASCT) followed by either fractionated total body irradiation with 8 Gy (TBI, n = 71) or non-TBI regimens (busulfan, n = 101, or treosulfan, n = 14) in combination with fludarabin (120 - 150 mg/m 2) from day -6 before ASCT. TBI-based conditioning was applied almost exclusively in patients aged < 60 years. Graft versus host-disease (GvHD) prophylaxis consisted of calcineurin inhibitors, mycophenolate and anti-T-lymphocyte globulin. In 180 out of 186 patients, blast clearance was assessed by cytologic and flow cytometric blast count via bone marrow (BM) aspirate in median 5 days after Melphalan and prior to continuation of the sequential conditioning therapy. Blast clearance was defined as cytologic BM blast count < 5% and < 0.1% BM cells with leukemia-associated immunophenotype in flow cytometry.
Results: Median pts age was 67 years (range 45 - 76 years) in the non-TBI group and 51 years (range 19 - 71 years) in the TBI group, respectively. Median follow up of surviving patients was 46 months. As expected, comorbidities and disease-specific factors varied between these groups. HCTCI scores > 3 points were observed in 14% of TBI pts compared to 32% in non-TBI pts ( p .01) and 45% versus 54% harbored adverse risk genetics according to European Leukemia Net (ELN) 2017 classification ( p .41), respectively. In the non-TBI group, 38% of the pts had a secondary or therapy-related AML compared to 20% of pts in the TBI group ( p .03). HLA-mismatched (<10/10 matched) donor grafts were transplanted in 21% (TBI) vs. 20% (non-TBI) of the pts. 185 of 188 pts received GCS-F mobilized stem cell grafts; 3 pts received a bone marrow graft.
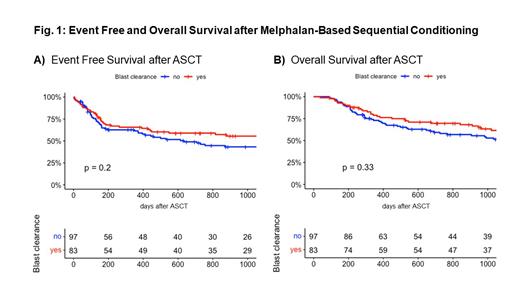
Kaplan-Meier estimates for overall survival (OS) with and without blast clearance at 2 years were 69.6% (95% CI 60.1-80.6%) vs. 58% (95% CI 48.8-69.1%, p .33), for event free survival (EFS, event defined as death or overt relapse) 58.9% (95% CI 49-70.8%) vs. 46.1% (95% CI 36.7-58.1%, p .2), respectively. We included the re-occurrence / persistence of defined molecular and cytogenetic markers or decreasing CD34 + bone marrow chimerism (< 95%) after transplant as measures for impending relapse as a modified EFS. The EFS including impending relapse was 49.8% (95% CI 40-62%) vs. 36.4% (27.7-47.8%) after 2 years. A non-significant trend with reduced relapse risk for those patients who achieved blast clearance after Melphalan ( p .1, HR 0.73) was observed. Most patients with impending relapse showed similar survival as patients without evidence of relapse after ASCT. In multivariate cox regression, age, HCTCI scores > 3 points, adverse risk according to ELN 2017 definitions and donor mismatch < 10/10 resulted in adverse prognosis. Especially, patients with mismatched donors displayed highly significant inferior outcomes. Both leukemic burden prior to ASCT as well as blast clearance after melphalan showed no significant correlation with clinical outcome parameters.
Conclusion: Sequential conditioning regimens followed by ASCT offer long-term survival for more than 50% of pts with active relapsed or refractory AML. Patients who did not achieve complete blast clearance during conditioning therapy showed a trend towards higher relapse risk after ASCT which did not result in inferior survival. This underscores the importance of close monitoring and early interventions such as accelerated immunosuppression tapering, preemptive donor lymphocyte infusions and targeted therapies in case of impending relapse after ASCT.
Disclosures
Mikesch:Pfizer: Honoraria, Other: Advisory Boards; Daiichi Sankyo: Honoraria, Other: Advisory Boards; Celgene: Honoraria, Other: Congress Support; Novartis: Honoraria; BeiGene: Honoraria; Jazz Pharmaceutics: Honoraria. Schliemann:Boehringer Ingelheim: Research Funding; AngioBiomed: Research Funding; Pfizer: Honoraria, Other; Roche: Honoraria; Novartis AG: Honoraria; Jazz Pharmaceuticals: Honoraria, Other, Research Funding; Bristol Myers Squibb: Honoraria, Other; AstraZeneca: Honoraria; Astellas Pharma Inc.: Honoraria; Laboratoires Delbert: Honoraria; AbbVie Inc.: Honoraria, Other. Lenz:Genmab: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Karyopharm: Consultancy, Membership on an entity's Board of Directors or advisory committees; Constellation: Consultancy, Membership on an entity's Board of Directors or advisory committees; ADC Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Miltenyi: Consultancy, Membership on an entity's Board of Directors or advisory committees; PentixPharm: Consultancy, Membership on an entity's Board of Directors or advisory committees; Sobi: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Immagene: Consultancy; Genase: Consultancy; Hexal/Sandoz: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Lilly: Consultancy; University Hospital Munster: Current Employment; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; NanoString: Membership on an entity's Board of Directors or advisory committees; BeiGene: Membership on an entity's Board of Directors or advisory committees; Morphosys: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Incyte: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BMS: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; AstraZeneca: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Bayer: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Gilead: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; F. Hoffmann-La Roche Ltd: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Stelljes:Gilead: Speakers Bureau; MSD: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Amgen: Membership on an entity's Board of Directors or advisory committees; Novartis: Speakers Bureau; abbvie: Speakers Bureau; Jazz: Speakers Bureau; medac: Other: Editorial and statistical support , Speakers Bureau.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal